Case Study: The Forsyth Institute
Project Management Brief
Brief
Design-product Systems brought critical project management discipline to help a small research clinic qualify candidates for inclusion in a weight-loss study whose eligibility requirements excluded 90% of applicants from participating. The study team had to scale several core study management operations to handle three times the normal subject volume in the project’s 12-week time frame.
DPS Scales Research Clinic Operations To Perform High-Volume Subject Acquisition
Design-product Systems provided the core study team with the resources and perspective to adapt their existing methods to meet this study’s outsized parameters. The subject population was three times that of the clinic’s typical study.
Advertising: Eligible subjects were women within certain age and Body Mass Index ranges who were not taking any long-term prescription medication. Surprisingly, these criteria excluded 75% of applicants! This required us to generate and field four times as many inquiries for every subject who participated. Typical advertising consisted of neighborhood flyering, contacting previous study participants, and advertising on forsyth.org and craigslist.org. In addition, we placed ads in subway cars, on Thomson CenterWatch, and in a suburban parents’ newspaper which generated many respondents in our target demographic.
Recruitment: Typically, a study coordinator could enroll subject populations for several small studies. New internal procedures, tools, team roles, and functions were needed to handle the high volume of calls and emails.
- Limited phone coverage created a backlog of voice mail messages. A four-extension phone chain was set up to ensure double coverage during business hours so that more inbound calls were answered.
- A shared clinic calendar allowed for simultaneous appointment scheduling, and coordination with the clinic’s other ongoing studies.
- Paper-based record keeping was replaced with a subject tracking database, which enabled the team to more quickly identify and recontact previously disqualified candidates when the study’s exclusion criteria were relaxed.
Patient Flow: With only twelve weeks in our original schedule (later expanded to sixteen), we had to run the clinic with unprecedented efficiency and teamwork.
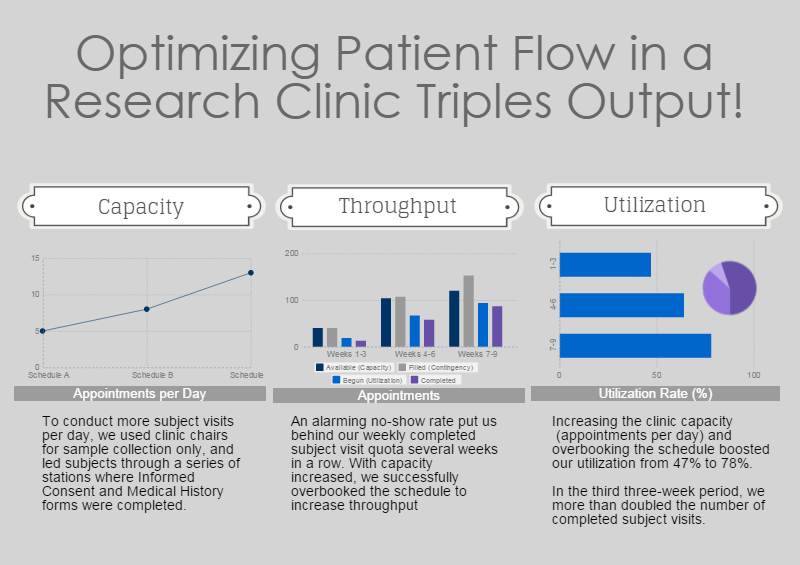
- Typically an entire subject visit was conducted in one of three available exam rooms. With a 90-minute study protocol almost evenly divided between data collection and sample taking, we conducted these activities in different locations, and thus handled more subjects simultaneously at different points in the protocol, almost doubling our capacity.
- An alarmingly high no-show rate created gaps in our clinic schedule and put us behind our weekly appointment quota several weeks in a row. With a revamped clinic schedule, we successfully double-booked appointments to offset the observed frequency of no-shows. This revealed major bottlenecks in our clinic operation that we resolved by reassigning tasks.
- An important detail specified in the approved study documentation required the study coordinator to leave the clinic frequently to keep study documents in compliance. This negatively impacted telephone coverage and patient flow. Obtaining approval to remove this requirement cut patient discharge time in half, and with improved telephone coverage, our weekly acceptance numbers increased.
Data Management and Reporting: We provided the client with weekly reports, and convinced them to relax the inclusion criteria to increase acceptance and throughput without impacting quality.
Project Outcomes
- 1,200 inquiries generated
- 315 subjects evaluated in clinic
- 32 eligible subjects were enrolled in a Phase 1 clinical trial
- Inclusion criteria was relaxed without affecting quality
- Clinical staff and resources were optimally utilized
- Study schedule and budget were increased by client
- Feedback from subjects and personnel was incorporated into operations and relayed to client
- Academic-industry partnership was maintained throughout the study
- Independent sample analysis resulted in published research
Like What You See So Far? Check out the
Partner
Package
Design-product Systems provided overall project management, team coordination, and analysis and troubleshooting.
- Hands-on activities included
- database development
- case report form (CRF) design
- patient flow analysis
- client reporting
- continuous process improvement
- Planning for study operations included
- call center staffing and supervision
- pre-screening by telephone and email
- subject tracking and recontact
- inclusion/exclusion reporting
- clinic calendaring
- schedule development